
Maart 2018
28
Targeted breeding technologies
– the way of the future
A
modern era of targeted crop breed-
ing is upon us. Traditional plant
breeding aims to change traits/
genes in a specific crop to obtain
the desired characteristics in the offspring.
This targeted trait is often linked to a specif-
ic change (mutation) in the parental plants’
genetic code (DNA), which the breeder
then attempts to develop progeny from,
containing target market characteristics.
Though this process seems relatively
straight forward it is not, since some traits
are ‘hidden’ (in the form of recessive genes),
while other traits are transferred to the prog-
eny in large groups (linked) that may include
undesirable traits as well (linkage drag).
Unwanted traits are also randomly trans-
ferred to the progeny, which means as the
number of desired traits increases, the
number of progeny required to obtain an
individual with all the desired traits and
development cost, increases dramatically.
This numbers game becomes even more
complicated when breeding with grain
crops. This is especially the case for wheat,
with its three large complex genomes, hav-
ing multiple copies of a single gene that
originated from the ancestry donor back-
grounds.
The breeding process always aims to pro-
duce cultivars faster and therefore currently
uses tools such as molecular selection
(marker-assisted breeding), embryo rescue
and double haploid generation and speed
breeding to accomplish this.
This ultimately results, after many years
(eight to twelve years) of breeding and se-
lection cycles (including traditional trait
screening and molecular selection), in the
release of higher yielding and adapted
cultivars.
New plant breeding tech-
nologies targets traits
better
However, what if a breeder could actu-
ally ‘target’ a desired trait with precision,
thereby transferring only the new desired
trait into an elite line without other un-
wanted characteristics? Well, some of the
new breeding technologies in the breeder’s
toolbox will now allow just that: The ability
to transfer a specific trait by targeting the
specific genetic code or gene region re-
sponsible for it.
These new plant breeding tools include a
wide variety of technologies, ranging from
directed nucleases for targeted mutagen-
esis to technologies that transfer the trait
of interest but does not result in permanent
DNA changes.
The tools in the new breeding toolbox that
are really making a huge impact are those
belonging to the directed nucleases group.
Nucleases are enzymes that can cut DNA.
Some of these nucleases recognise and
cut only specific DNA sequences (e.g. me-
ganucleases), while others use engineered
proteins to target specific DNA for cleav-
ing. The usefulness of meganucleases are
limited since they can only target and cut at
their specific DNA recognition sequences,
which will very rarely be within the target
trait region desired by the breeder. These
technologies require expensive, time-
consuming protein engineering skills by
experienced individuals. All these directed
nucleases have been around for a number
of years, but not widely adopted due to their
limitations. This all changed with the discov-
ery of the CRISPR/Cas system – a system
awarded the
Science Discovery of the Year
in 2015.
CRISPR/Cas: Get familiar
with it
CRISPR/Cas9 (clustered regularly inter-
spaced short palindromic repeat)-Cas9 is a
multipurpose system for targeted genetic
engineering that uses a ‘guide molecule’
(guide RNA) to direct a DNA nuclease (Cas9
or other similar endonuclease) to a specific
target site where it cuts the DNA in a spe-
cific manner.
The guide can easily and quickly be chang-
ed and synthesised as DNA (or RNA) using
current technologies, making this system
not only cheaper, but also faster to imple-
ment,with lessexpertiserequired.Theusage
of the system is restricted by a Cas nuclease
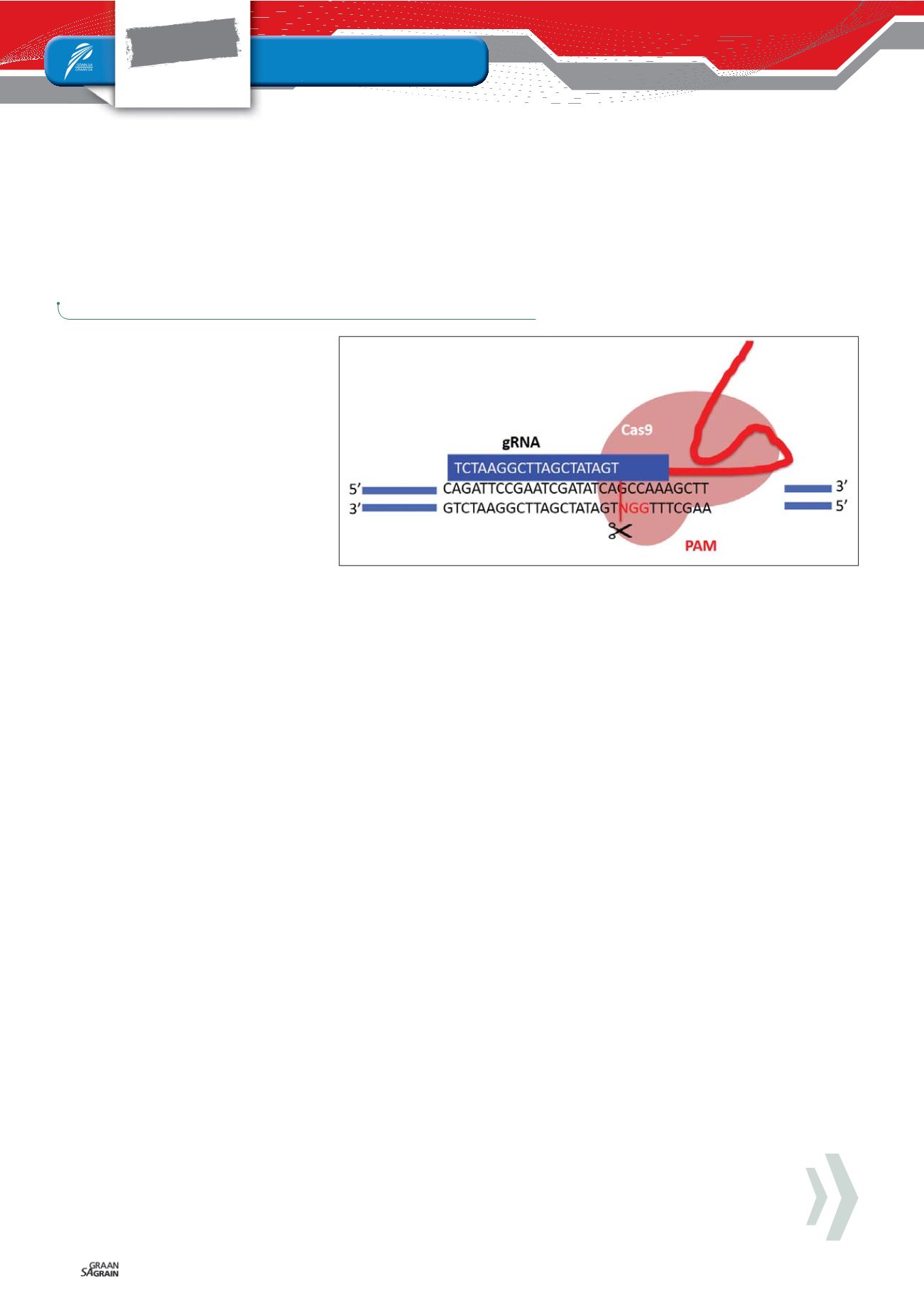
specific protospacer adjacent motif (PAM)
– a short, Cas enzyme specific sequence, re-
quired to be in the target DNA for successful
binding and cutting (
Figure 1
).
This PAM sequence differs between dif-
ferent Cas nucleases, thereby increasing
possible cutting sites and allowing easier
targeting of a different desired DNA se-
quence. The availability of complete ge-
nome sequence, target gene sequence/
mutation and appropriate in vitro (tissue
culture) delivery system, are some of the
major limitations of the system currently.
FOCUS
Seed
Special
DR SCOTT SYDENHAM,
ARC-Small Grain, Bethlehem and
DR DIRK SWANEVELDER,
ARC-Biotechnology Platform, Onderstepoort
Figure 1: The CRISPR associated nucleases (Cas9) binds to a guiding molecule, the guide RNA
(gRNA), which has a complementary sequence to the DNA being targeted in the genome. This ribo-
nucleoprotein (Cas9-gRNA complex) moves along the DNA of the organisms in search of the comple-
mentary target sequence. Once found, the Cas9-gRNA ribonucleoprotein complex binds to the DNA
in the presence of a PAM (protospacer adjacent motif) sequence (in red – NGG), thereby aligning the
nuclease to cut the DNA at a specific site. By changing the gRNA’s sequence (white text in blue box)
another site in the DNA could easily be targeted. Multiple gRNAs allow multiple DNA sites being
targeted at once – though efficiency does decrease.
















